Megakaryon has adopted the platelet production techniques developed in The University of Tokyo and Kyoto University. The researchers discovered a method to proliferate megakaryocytes and induce platelet production which forms the basis of mass production of platelets from iPS cells. Megakaryon has obtained exclusive rights to use the patents of these techniques and side-by-side is conducting research to enable the mass production of platelets.

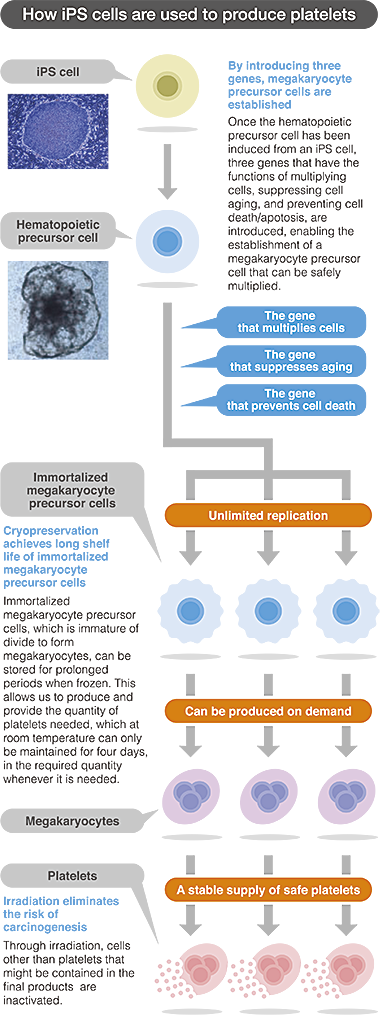
Our company, “Megakaryon” was named after megakaryocytes. Megakaryocytes are the precursors of platelets and the key to producing platelets from iPS cells are dependent on the development of megakaryocytes from iPS cells. As freezing impairs the platelet function, it can be stored only at room temperature and the shelf-life is limited to four days. This represents a bottleneck in producing a stable supply of platelets.
The patented technique involves the incorporation of three genes to the iPS cells to establish an immortalized megakaryocyte cell line that can be stored frozen and can successfully produce functional platelets when cultured. With this technological breakthrough as the cornerstone, Megakaryon Corporation is incorporating further improvements to the process to develop the techniques that will enable mass production of functional platelets on demand.

We aim to achieve high degree of safety for our platelet formulation. In addition to mass production, our technology has two additional advantages, both of which concern safety of the product. First, the process does not involve any donor-associated pathogenic risk of bacterial or viral infection. Since the technology facilities the mass production of platelets through propagation of cells in a sterile environment, the safety of the master cell is guaranteed, and the manufacturing is conducted in strictly controlled, pathogen-free environment with multiple checkpoints for quality control. Second, there is no risk of carcinogenicity. Carcinogenicity is a major concern in regenerative medicine applications where iPS cells are used. In simpler terms, platelets lack nucleus and during platelet formulation process, the product is gamma irradiated to destroy any viable cells that may be present, making the final product a safe and non-carcinogenic one.

Currently, it is very difficult to ensure a continuous supply of HLA-matched platelets for patients with rare HLA types. Our technology aims to collect the iPS cells from donors with rare HLA types and use these cells to prepare megakaryocytes that can then be stored frozen in a cell bank and propagated in large quantities when required, thereby ensuring a constant supply of platelets for patients with rare HLA types.
Megakaryon has signed contracts granting the company exclusive rights to use the patents (listed below) related to platelet production from iPS cells that was developed in The University of Tokyo and Kyoto University.
Some of these patents have already been issued in multiple countries. The company plans to reinforce the rights surrounding these patents, and strategically employ these patents for further inventions and additional patented techniques/products.